Next page: Ask our AI any related question
In the United States, one of the most consistently growing age groups is the 65+ demographic, driven by declining birth rates and breakthroughs in medical science and technology. It is projected that by 2060, this segment will comprise nearly 25% of the entire U.S. population.
By the year 2034, for the first time ever, the number of elderly adults is expected to surpass the number of children, significantly affecting the availability of both family and community care resources. With an aging population, there will be an increased need for preventive health measures designed to prolong the healthy lifespan and ensure efficient use of available resources.
By 2060, nearly one in four Americans is projected to be an older adult.
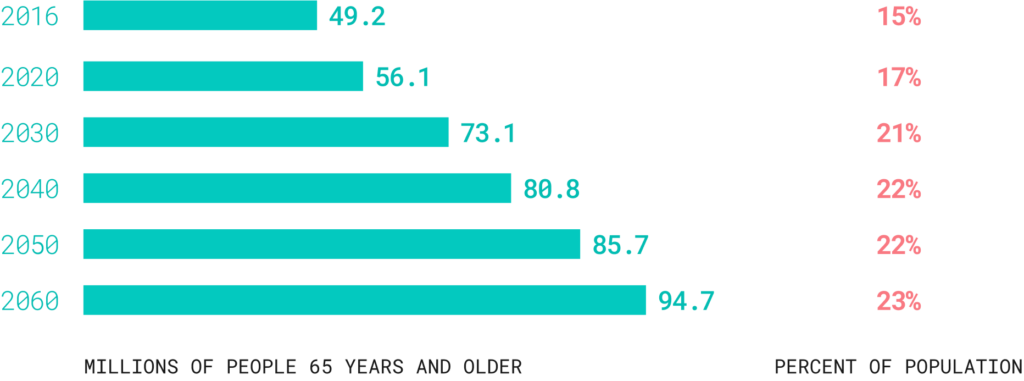
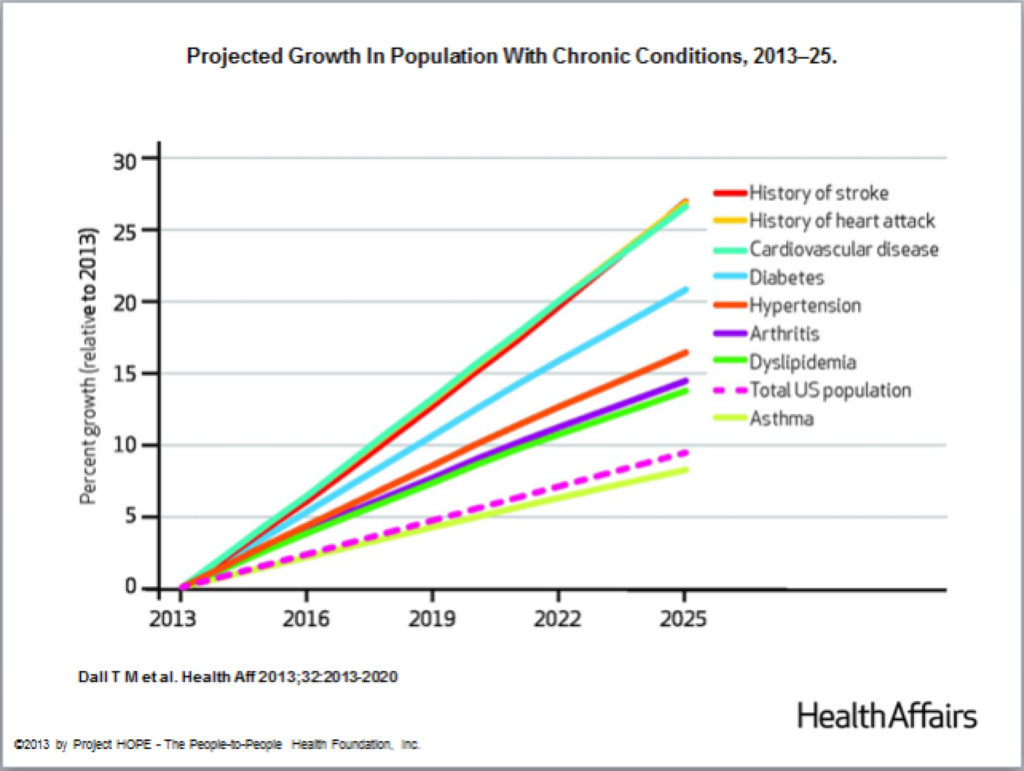
As life expectancy increases, so too does the prevalence of age-related diseases and degenerative conditions, which escalates healthcare costs and the overall burden of care. Currently, 80% of older Americans are affected by at least one chronic illness, with many suffering from two or more. These statistics are expected to rise as the population ages.
Modern medical practices typically address these conditions individually, focusing on managing symptoms or slowing the progression of diseases like osteoarthritis, cardiovascular disease, and diabetes. This segmented approach to treatment places additional strain on the healthcare system as it manages these long-term conditions. A more revolutionary approach would be to tackle the fundamental processes of aging itself to enhance the overall health span.

The impact of aging is readily observable in the body and its organ systems, stemming from alterations at the cellular and molecular levels. As cells age, their capacity to perform essential homeostatic functions—such as cell division, protein synthesis, metabolism, and cell-to-cell communication—gradually decreases.
Additionally, the cumulative wear-and-tear from years of life and environmental stressors significantly disrupts both the composition and the structural integrity of cellular DNA. These changes are collectively described by the Nine Hallmarks of Aging.
As cells progress in age, they begin to exhibit these Nine Hallmarks, moving towards a state of senescence, characterized by a loss of cell division activity, and ultimately leading to cell death. This transition affects not just the aging cells but also has deleterious effects on neighboring cells.
Senescent cells emit a variety of harmful signals, known as the senescence-associated secretory phenotype (SASP), which amplifies pro-inflammatory responses and can detrimentally alter the local cellular environment.
Developing therapies that can interrupt this path towards cellular senescence and restore functional cell operations offers the possibility of significant, broad-spectrum health improvements, potentially transforming our understanding of and approaches to managing aging.
Several theories explore the fundamental mechanisms behind aging, yet a comprehensive explanation suggests it stems from widespread alterations within the cellular epigenome. This complex network, encompassing DNA methylation sites, histone modifications, large-scale heterochromatin areas, and topologically associated domains, serves as a critical upstream regulator that controls gene expression within cells.
As individuals age, their cells experience an accumulation of epigenetic changes leading to dysregulation and dysfunction. This epigenetic imbalance causes disruptions in both intracellular gene expression and extracellular signaling, culminating in the multifaceted dysfunction of cells and tissues recognized as aging. Interventions that rectify epigenetic abnormalities can significantly improve the biological markers associated with the nine hallmarks of aging and potentially address various age-related diseases.
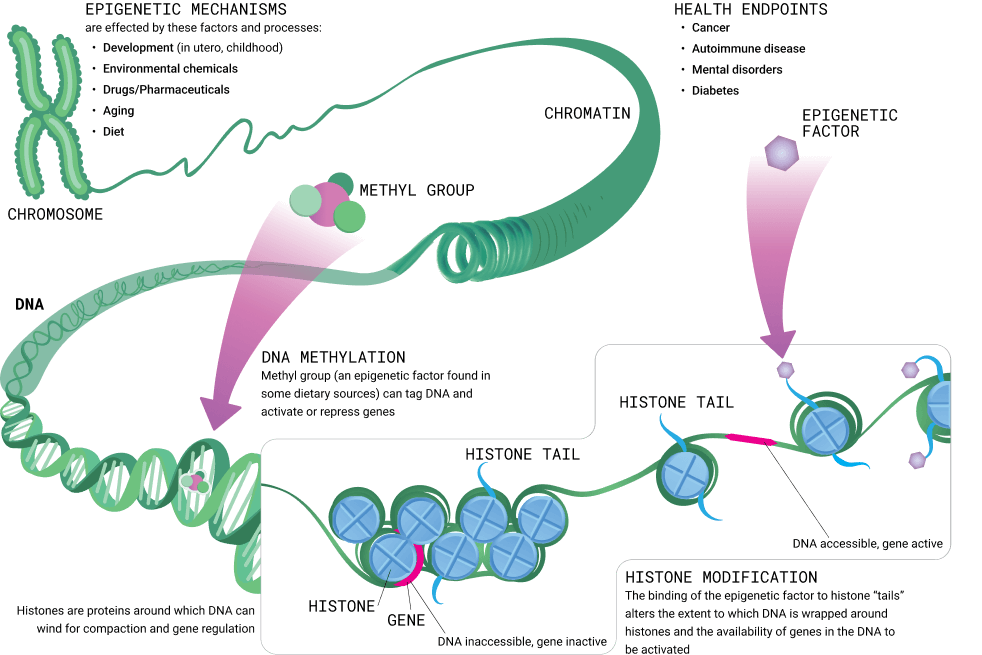
The epigenome acts as a crucial framework within every cell, providing a structure upon which DNA rests, thereby regulating gene expression and maintaining cellular balance. While every cell possesses the same DNA “book,” the epigenome highlights specific gene segments necessary for expression in various cells. This selective process facilitates the evolution of diverse, specialized cells from a common progenitor, the embryonic stem cell.
Proper epigenomic functioning is essential for appropriate gene activity in response to environmental stimuli. Several factors, including medications, diet, developmental stages, and aging, can influence epigenomic dynamics. There is a strong link between a cell’s age and its epigenomic state, suggesting that the epigenome effectively chronicles a cell’s age. This complex interaction ensures that each cell type is finely tuned and distinctly functional, illustrating the epigenome’s vital role in biological processes.
The reprogramming process in iPSCs has been leveraged in numerous studies to target specific age-related biological pathways, such as enhancing nutrient sensing, boosting mitochondrial biogenesis, and promoting the clearance of degraded proteins. iPSCs have been successfully derived from cells of volunteers well into their centenary, demonstrating that reprogramming factors can revert aged cells back to a youthful, embryonic stem cell-like state and effectively erase the biological markers of aging. Despite these promising in vitro results, direct in vivo application of these factors in animal models has led to complications, including the formation of tumors, thus posing significant limitations on their direct therapeutic use.
One experimental approach that has shown potential involves precisely controlling the expression duration of reprogramming factors, yielding promising anti-aging results in animal models. The challenge remains to harness the power of cellular reprogramming within safe and ethical boundaries to develop viable anti-aging therapies for human use.
Despite inherent challenges, cellular reprogramming and iPSC (induced pluripotent stem cell) technology hold promising potential for use in regenerative and anti-aging therapies. However, these technologies must be applied with caution: they need to 1) maintain tissue organization and functionality, and 2) avoid increasing the risks of carcinogenesis or mutagenesis.
To this end, three primary strategies are being developed for therapeutic applications:
This involves deriving iPSCs from patient cells, growing them in vitro, and then retransplanting them back into the patient
iPSCs are also grown from patient cells in vitro, but instead of retransplantation, the bioactive factors and proteins they produce are harvested and used to create personalized therapies
This strategy focuses on optimizing the targeted delivery and the duration of reprogramming factors directly to adult tissues
In vivo reprogramming involves directly converting one type of mature cell into another within a living organism, bypassing the stem cell stage. This is done by introducing specific genes or factors that reprogram the target cells into desired cell types directly within the body. This method has the potential for regenerative medicine directly at injury or disease sites.
On the other hand, iPSC reprogramming (induced pluripotent stem cell reprogramming) involves taking mature cells from an organism and reprogramming them in a lab into pluripotent stem cells, which can then differentiate into any cell type. This process involves reverting cells to a stem cell-like state outside the body before inducing them to become the desired cell type.
The key difference is that in vivo reprogramming skips the pluripotent stage and happens inside the body, whereas iPSC reprogramming involves creating stem cells in a laboratory setting and then differentiating them into specific cells.
With ex vivo applications, cells can be meticulously monitored outside the body, allowing for accurate derivation of patient-specific iPSCs that can be tailored to generate individualized treatments. This process ensures that any cellular anomalies, particularly potential oncogenic changes, are identified and addressed before the cells are reintroduced into the patient’s body.
Furthermore, because these cells originate from the patient’s own tissue, they are less likely to trigger an immune response upon reintroduction, offering a significant advantage over other biologic therapies. However, these interventions require substantial resources, making them not universally applicable but highly specialized.
Conversely, in vivo applications offer a broader potential application by enabling the fine-tuning of reprogramming factor delivery and action directly within the body’s tissues. This approach aims to recalibrate the epigenome in a controlled manner, akin to repairing a damaged structure — heating it to smooth out the imperfections and then cooling it to restore its original function, without breaking down its fundamental structure as occurs in iPSC reprogramming. Such finely controlled in vivo reprogramming could potentially revolutionize the treatment of age-related conditions by rejuvenating cells and restoring their youthful functionality without regressing them completely to a pluripotent state.

The challenge with in vivo reprogramming lies in establishing precise controls over the delivery and duration of treatment. The objective is to harness this technology to introduce a controlled degree of plasticity back into the epigenome, thus rejuvenating cellular function. This represents a transformative approach in the 21st-century therapeutic landscape, shifting from traditional pharmaceutical interventions that typically target single pathways to a method that offers a holistic improvement of global cell behavior.
As populations around the world and specifically in the USA continue to age, the need for innovative therapies that extend healthspan becomes increasingly critical. Numerous emerging pharmacological and biological treatments are being developed to mitigate the effects of age-related diseases by targeting the fundamental hallmarks of aging. Among these, therapies that modify the epigenomic aspects of aging hold significant promise due to their potential to directly influence the underlying causes of age-related decline.
Rejuvenation through reprogramming technologies has shown promising results in preclinical studies, demonstrating substantial functional improvements in aged cells across various types. The advancement of these therapies into clinical trials is highly anticipated, offering hope for transformative interventions that can significantly enhance the quality of life for the aging population.
Previous page: Background
Request password by email: info@ananda-labs.com